2025
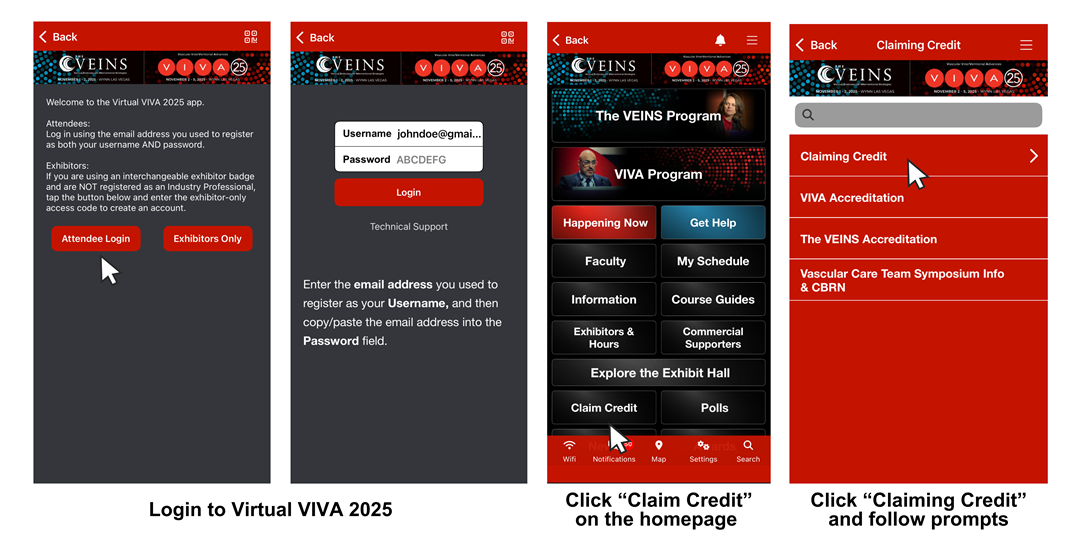
- Download the Virtual VIVA 2025 app from the App Store or Google Play - Download here.
OR access the accreditation platform on your desktop - Claim credit here. - Login to Virtual VIVA 2025 using your registration email and password. If logging in to Virtual VIVA 2025 for the first time, use your registration email address as both your username AND password.
- Select a conference.
- Click "claim credits" on the conference homepage.
- Click "claiming credits" and follow the prompts on screen to complete the 2025 evaluation and claim credit.
Using the mobile app:
2024
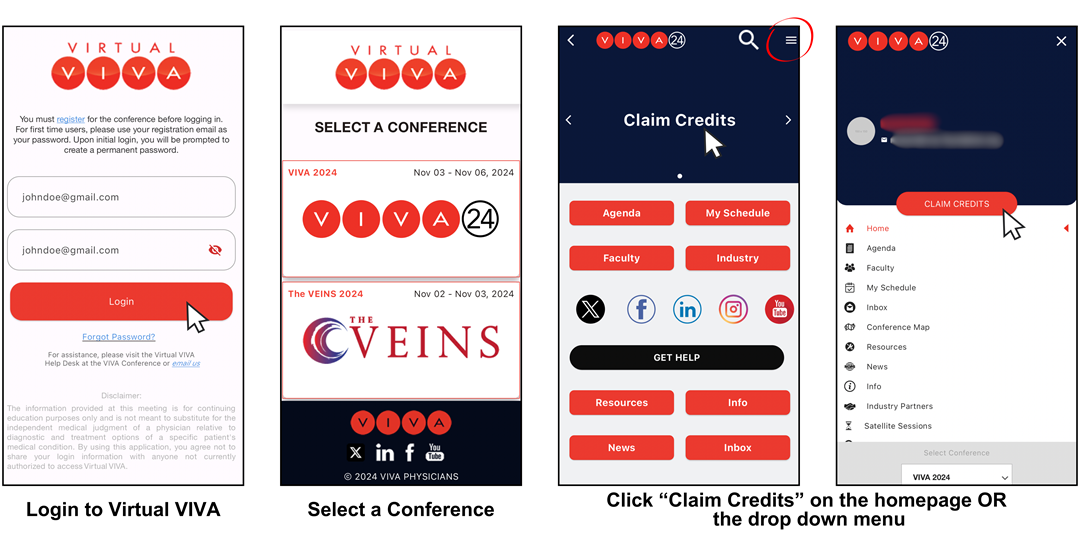
- Download the Virtual VIVA app from the App Store or Google Play
- Login to Virtual VIVA using your registration email and password. If logging in to Virtual VIVA for the first time in 2024, use your registration email address as both your username AND password. You will then be prompted to create a new password.
- Select a conference.
- Click "claim credits" on the conference homepage or in the dropdown menu.
- Follow the prompts on screen to complete the 2024 evaluation and claim credit.
Please direct all CME-related questions to cme@viva-foundation.org.


